Topic Content:
- Definition of Diffusion
- Factors that Affect the Rate of Diffusion
- Experiment to Demonstrate Diffusion in Liquids
- Biological Significance of Diffusion
What is Diffusion?
Diffusion is defined as the movement of molecules of a substance from a region of higher concentration to a region of lower concentration down a concentration gradientA concentration gradient occurs in a solution or gas when the concentration of particles is higher in one area than in another. More.
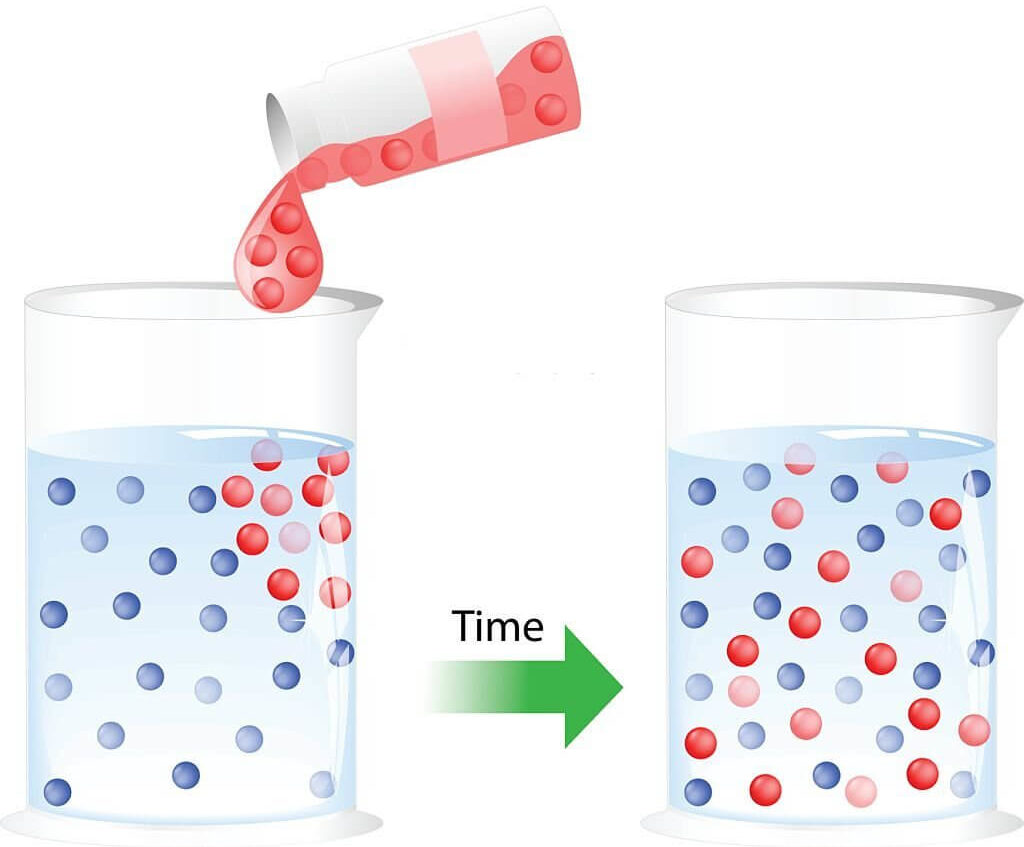
A concentration gradient occurs when the concentration of particles is higher in one area than in another.
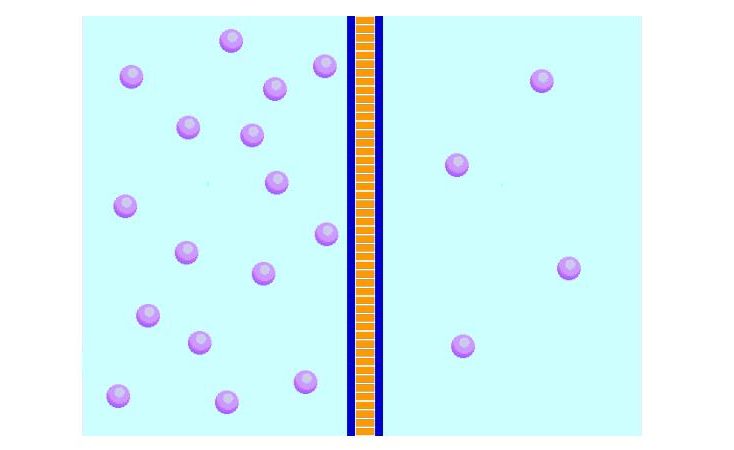
Diffusion is an important process for living things; it is how substances move in and out of cells.
Factors that Affect the Rate of Diffusion:
You are viewing an excerpt of this Topic. Subscribe Now to get Full Access to ALL this Subject's Topics and Quizzes for this Term!
Click on the button "Subscribe Now" below for Full Access!
Subscribe Now
Note: If you have Already Subscribed and you are seeing this message, it means you are logged out. Please Log In using the Login Button Below to Carry on Studying!



Responses