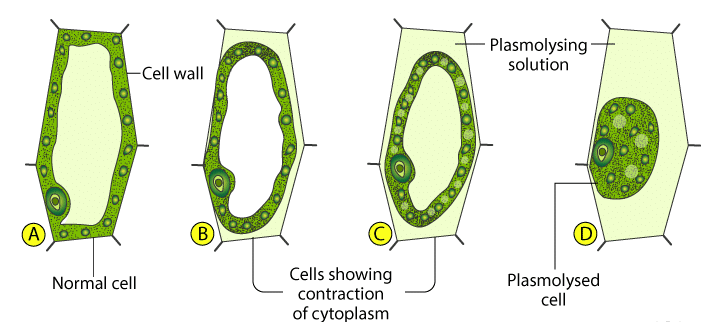
Plasmolysis:
When a living plant cell is placed or surrounded in a sugar or salt solution, a more concentrated or hypertonic solutionA hypertonic solution refers to a solution containing a higher solute concentration in comparison to the solute concentration in another solution, across a semipermeable membrane. More than the cell sap, water will be lost from the cell to the stronger solution resulting in exosmosis.
When the cell is left in the hypertonic solution for a long time, it will result in the pulling away of the cytoplasm from the cell wall, consequently, the cytoplasm will shrink and the cell wall will collapse. At this stage, we may say the cell is plasmolyzed.
Plasmolysis is the shrinking of the cytoplasm away from the cell wall when plant cells are immersed in a hypertonic solution.
Turgidity:
However, if a plant cell is placed in a hypotonic solutionHypotonic solution refers to a solution containing a lower solute concentration in comparison to the solute concentration in another solution, across a semipermeable membrane. Such a solution has a decreased solute... More, it behaves differently; water enters the cell by osmosis due to the fact that the cytoplasm solution is stronger than the water. This is called endosmosis.
As water enters the cell the vacuole increases in size and pushes the cell contents against the cell wall. The cellulose cell wall prevents overstretching of the cell by exerting an opposing pressure preventing the entry of more water. In this state the cell becomes turgid.
Turgidity is important in land plants because it makes them firm and gives support. On the other hand, if the plant is not supplied with water, it will die when all its cells become plasmolysed.
Flaccidity:
When the plant cell is placed in an isotonic solutionAn isotonic solution is when two solutions, separated by a semipermeable membrane, have equal concentrations of solutes and water. More, there is no net flow of water toward the outside or inside. When the flow of water into the cell and out of the cell exists in equilibrium then the plant cells are said to be flaccid.
You are viewing an excerpt of this Topic. Subscribe Now to get Full Access to ALL this Subject's Topics and Quizzes for this Term!
Click on the button "Subscribe Now" below for Full Access!
Subscribe Now
Note: If you have Already Subscribed and you are seeing this message, it means you are logged out. Please Log In using the Login Button Below to Carry on Studying!



Really helpful
Thanks alot . This helped at the last minute