Topic Content:
- Measurement of Acidity and Alkalinity
- Universal Indicator
- Acid-Base Indicator
- Common Indicators and their Colours in Acidic, Basic and Neutral Medium:
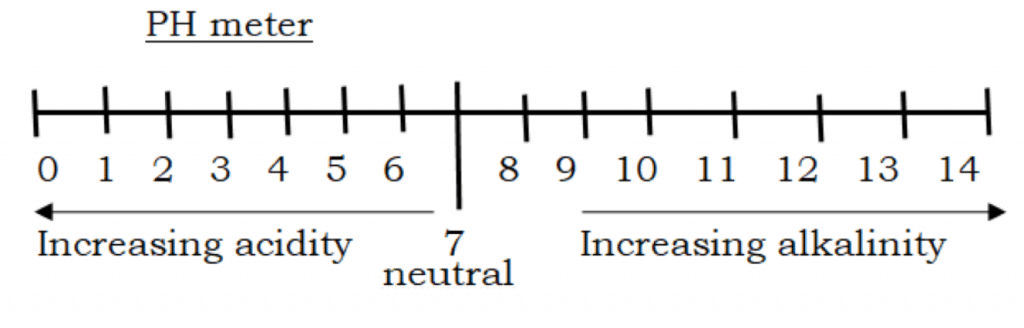
The acidity and alkalinity of a solution can be measured using a pH scale. The pH value of a solution is a measure of the degree of acidity or alkalinity of the solution. It is measured by an instrument or device called a pH meter.
The pH scale is numbered from 0 – 14. A solution with a pH value of 7 is neutral. pH value less than 7 is acidic. pH value greater than 7 is alkaline.
Acidity increases with decreasing pH, while alkalinity increases with increasing pH values. the pH of a solution can be measured with a pH meter or by the use of a universal indicator.
Universal Indicator:
A universal indicator is a mixture of dyes. It is composed of many indicators such as methyl orange, phenolphthalein, and methyl red. It shows a wide range of colours in the solution depending on the pH value of the solution.
Universal indicator is available either as a solution or impregnated into filter paper known as text paper. It exhibits various colours from red to orange, yellow, green, blue, indigo, and violet (ROYGBIV).
Universal indicators can be used to indicate the degree of acidity or alkalinity of a solution.
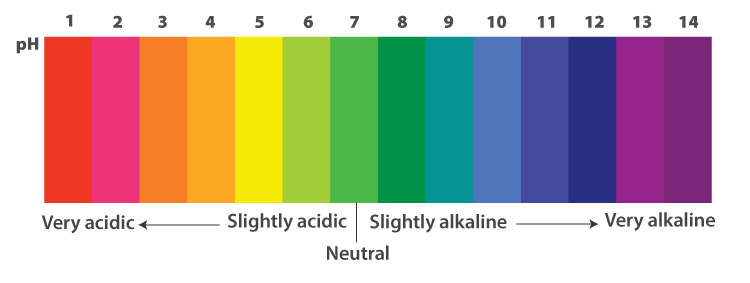
| pH Range | Colours with drops of Universal Indicator | Acidic or Basic |
| 1-3 | Red | Strongly Acidic |
| 4-5 | Orange | Weakly Acidic |
| 6 | Yellow | Very weakly Acidic |
| 7 | Green | Neutral |
| 8 | Blue | Very weakly Basic |
| 9-10 | Indigo | Weakly Basic |
| 11-14 | Violet | Strongly Basic |
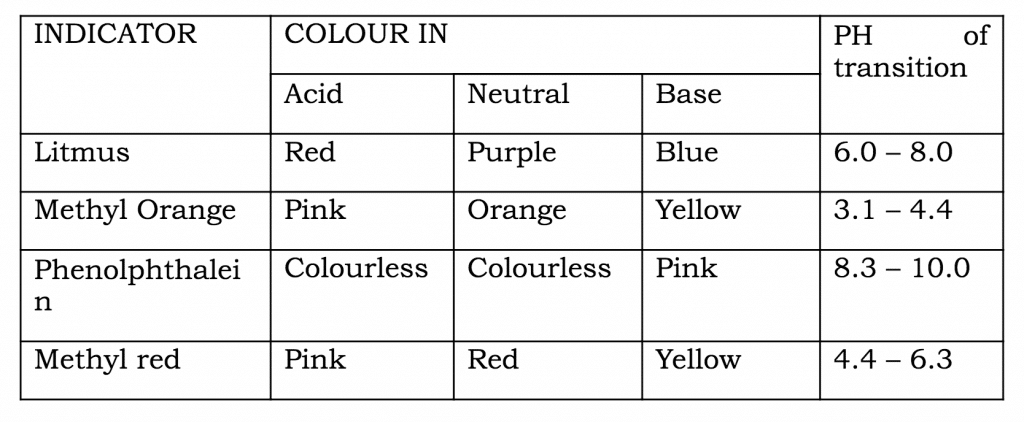
Acid-Base Indicator:
Indicators are organic acids or bases which show different colours in acidic and basic mediums.
Common Indicators and their Colours in Acidic, Basic and Neutral Medium:



Responses