Diffusion Rates of Ammonia (NH3) and Concentrated Hydrochloric Acid (HCl)
Topic Content:
- Diffusion Rates of Ammonia (NH3) and Concentrated Hydrochloric Acid (HCl)
- Theory Questions and Answers
Experiment: Diffusion Rates of Ammonia (NH3) and Concentrated Hydrochloric Acid (HCl)
(N = 14, H = 1, Cl = 35.5) NH3 = 17, HCl = 36.5
Apparatus:
Glass tube with open ends, cotton wool.
Procedure:
Soak a small quantity of cotton wool in a small solution of Ammonia. Place the soaked cotton wool at one open end of the dry glass tube.
Soak another cotton wool in a small quantity of concentrated hydrochloric acid.
Place it at the other open end of the glass tube.
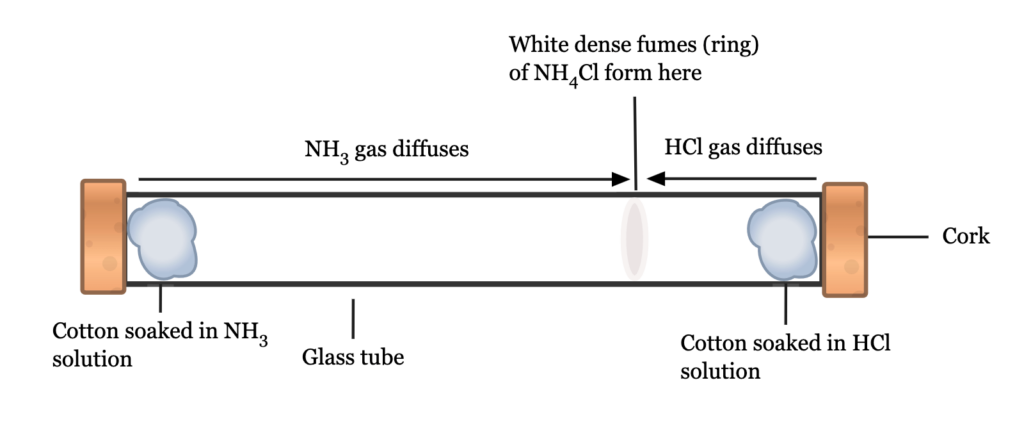
Observation
You are viewing an excerpt of this Topic. Subscribe Now to get Full Access to ALL this Subject's Topics and Quizzes for this Term!
Click on the button "Subscribe Now" below for Full Access!
Subscribe Now
Note: If you have Already Subscribed and you are seeing this message, it means you are logged out. Please Log In using the Login Button Below to Carry on Studying!



Responses