Experiment to Determine Apparent Cubic Expansivity of Liquid
Topic Content:
- Experiment to Determine Apparent Cubic Expansivity of Liquid
Experiment to Determine Apparent Cubic Expansivity of Liquid:
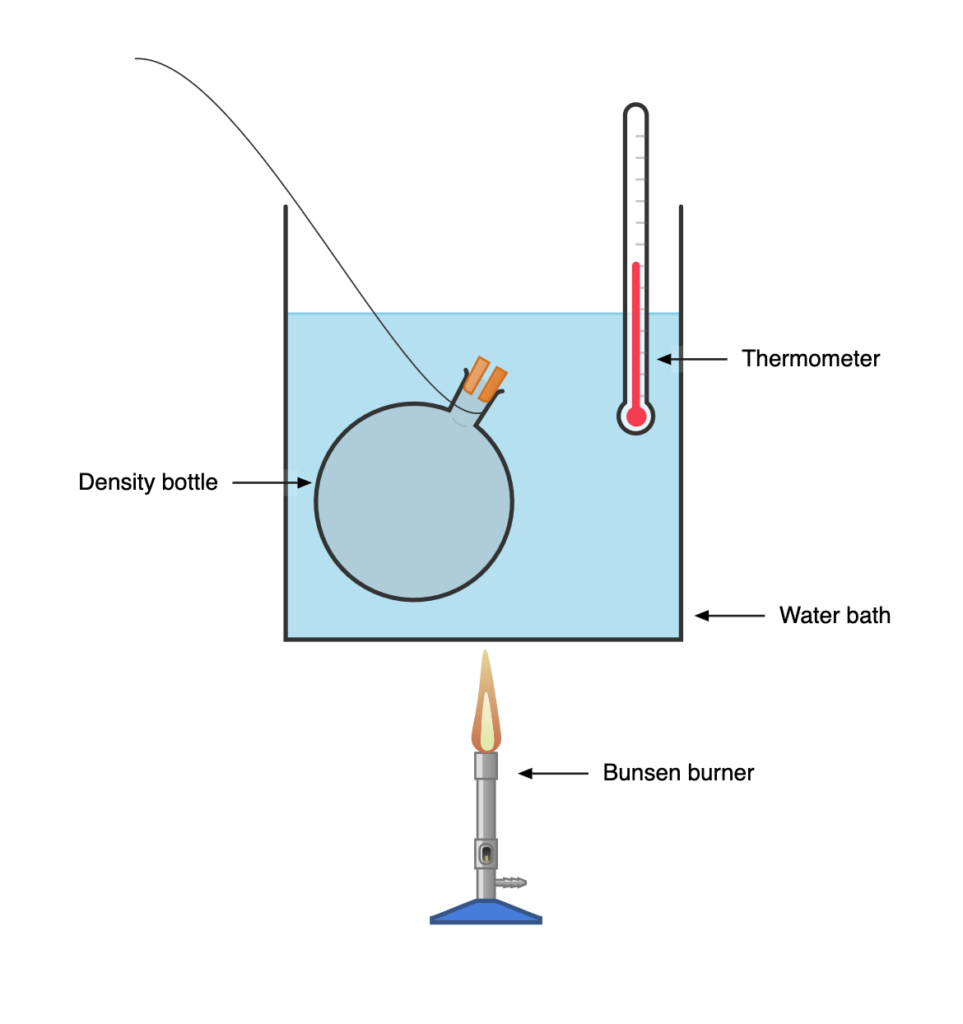
- Weigh a clean dry densityDensity is the measurement of how tightly a material is packed together i.e. how closely the particles are packed in the material. The tighter the material is packed the more its... More bottle and record mass, M1.
- Fill the bottle with liquid whose apparent cubic expansivity is required, replace the stopper, and dry
- Weigh the bottle and liquid, M2
- Suspend the density bottle in a water bath and record the initial temperature of the water bath, \( \scriptsize \theta_1\)
- Heat the water until it reaches boiling point, some liquid will be expelled through the orifice (opening) of the bottle stopper.
- Continue heating the water bath until no liquid is expelled from the space around the stopper.
- Note and record the final temperature of the water bath, \( \scriptsize \theta_2\)
- Remove the bottle from the bath and reweigh, M3
The Apparent cubic expansivity is calculated as follows:
Mass of empty bottle = M1
Mass of bottle and liquid = M2
Final mass of bottle and liquid = M3
Initial temperature of water = \( \scriptsize \theta_1\)
Final temperature of water = \( \scriptsize \theta_2\)
Mass of liquid expelled = M2 – M3
Mass of remaining liquid = M3 – M1
Apparent expansivity = \(\scriptsize γ_α\)
Since mass is proportional to volume,
Apparent cubic expansivity,
\( \scriptsize γ_a = \normalsize \frac {mass\: of\: liquid\: expelled}{mass\: of\: liquid\: remaining \: \times \:temperature\: rise}\)\( γ_a = \frac {M_2 \:- \:M_3}{(M_3\: – \:M_1) \: \times \: (\theta_2\: – \:\theta_1) }\)
Example 1.3.1:
A density bottle contains 44.25 g of a liquid at 0°C and 42.02 g at 50°C. Calculate the real cubic expansivity of the liquid. (Linear expansivity of glass = 1.0 × 10-5K-1)
Solution
You are viewing an excerpt of this Topic. Subscribe Now to get Full Access to ALL this Subject's Topics and Quizzes for this Term!
Click on the button "Subscribe Now" below for Full Access!
Subscribe Now
Note: If you have Already Subscribed and you are seeing this message, it means you are logged out. Please Log In using the Login Button Below to Carry on Studying!



can someone please explain the third example, its very confusing
This is very helpful and I aced my test