Topic Content:
- Complex Ionic Equation
Balancing complex ionic equations depends on the medium of reaction whether acidic or alkaline (basic) medium. They are called complex equations because they contain polyatomic ions.
In an acidic medium, the following steps must be considered;
1. Write down the oxidation number of each element or ion present in the medium
2. Divide the equation into two half-cell equations i.e. oxidation half-cell and reduction half-cell equation.
3. Balance each half-cell equation thus:
(a) To the reduction half-cell equation add H+ ions and H2O to the reactants and products respectively. Then balance the atoms present using appropriate coefficient numbers and finally balance the changes using the appropriate number of electrons.
(b) To the oxidation half-cell equation balance the atoms present using appropriate coefficient numbers and then balance the changes using an appropriate number of electrons.
4. Multiply each half-cell by using the coefficient of electrons to balance electron gain or loss
5. Add the two half-cell equations in (step 4) to get the NET EQUATION; which is the balanced complex ionic equation. (the electrons cancel out)
Example 8.2.1:
Balance the following equations for reactions occurring in an acidic medium.
(a) MnO–4(aq) + Sn2+(aq) → Mn2+(aq) + Sn4+(aq)
(b) Cr2O72-(aq) + Pb2+(aq) → Cr3+(aq) + Pb4+(aq)
Solution
(a) MnO–4(aq) + Sn2+(aq) → Mn2+(aq) + Sn4+(aq)
Step I:
MnO–4 = -1
Mn + [4 × (-2)] = -1
Mn – 8 = -1
Mn = +7
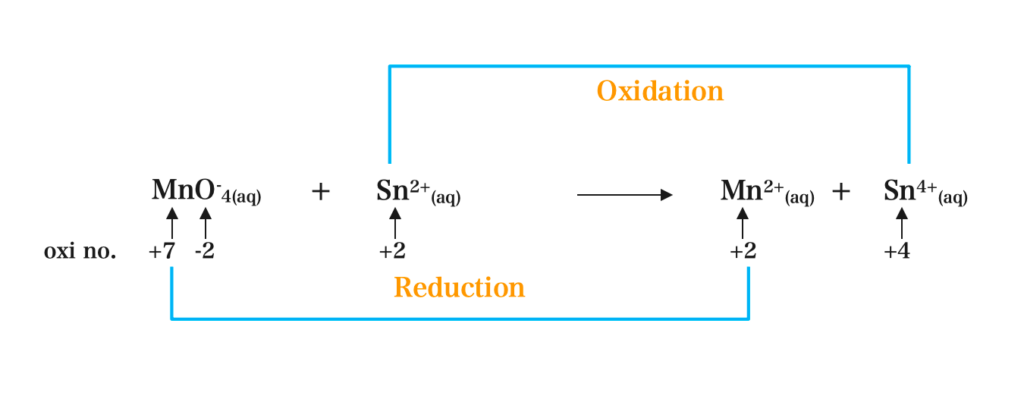
Step II:
You are viewing an excerpt of this Topic. Subscribe Now to get Full Access to ALL this Subject's Topics and Quizzes for this Term!
Click on the button "Subscribe Now" below for Full Access!
Subscribe Now
Note: If you have Already Subscribed and you are seeing this message, it means you are logged out. Please Log In using the Login Button Below to Carry on Studying!



Responses