Topic Content:
- Electronic Configuration and Periodic Table
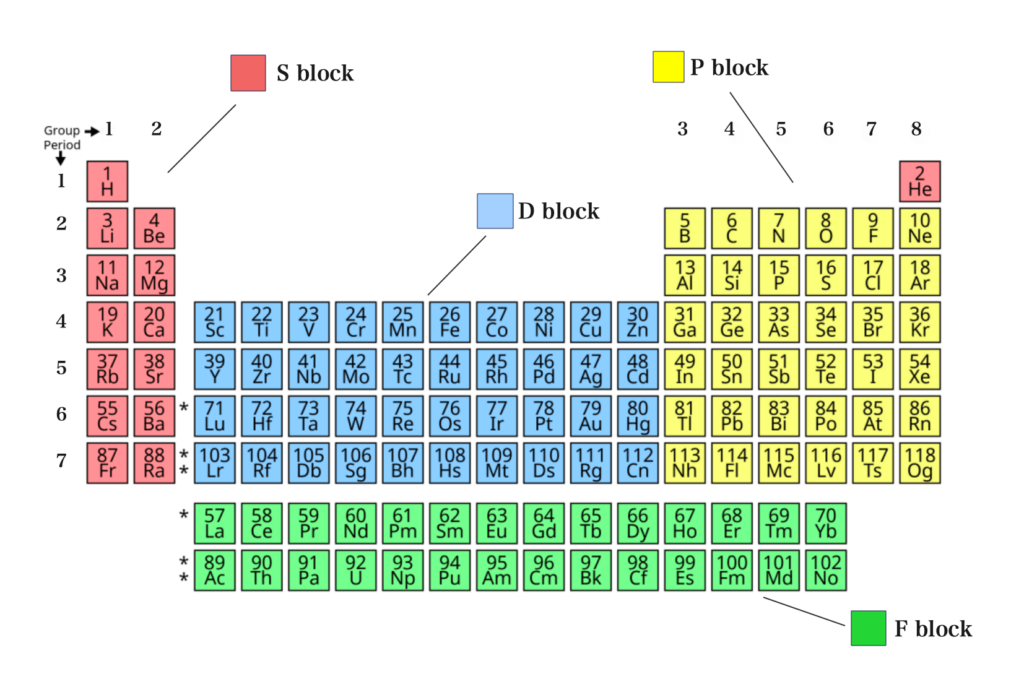
In the periodic table, elements are grouped from groups 1 – 7 and 0.
In writing electronic configuration, energyEnergy is the ability to do work. Energy exists in several forms such as heat, kinetic or mechanical energy, light, potential energy, and electrical energy. Units of Energy: The SI unit... More levels are used, which correspond to the filling of K, L, M, and N shells. In every energy level, there exist sub-levels which represent the filling of s, p, d & f orbitals. The following electronic configuration represents the elements in the outermost shell of each group.
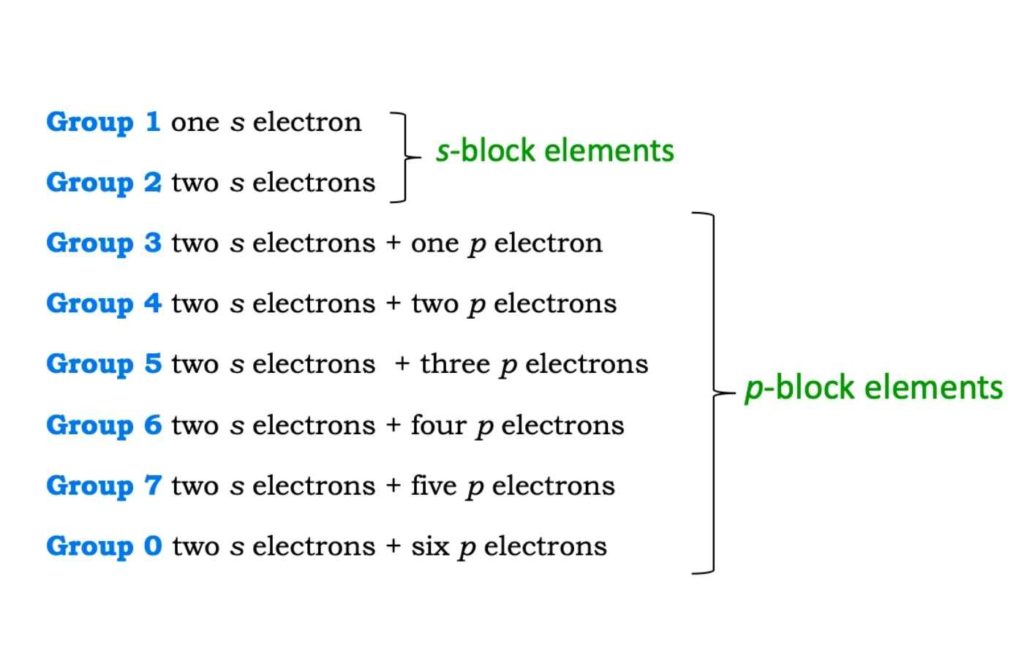
Groups 1 and 2 are the s-block elements. They are the alkali and alkaline earth metals. They are the most reactive metals. Groups 3 to 7 and 0 form the p-block elements.
You are viewing an excerpt of this Topic. Subscribe Now to get Full Access to ALL this Subject's Topics and Quizzes for this Term!
Click on the button "Subscribe Now" below for Full Access!
Subscribe Now
Note: If you have Already Subscribed and you are seeing this message, it means you are logged out. Please Log In using the Login Button Below to Carry on Studying!



Responses