Topic Content:
- Meaning of Latent Heat
- Specific Latent Heat of a Substance
- Specific Latent Heat of Fusion
What is Latent Heat?
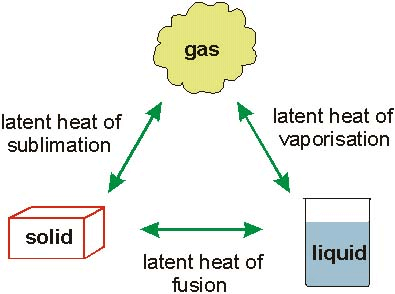
Latent heat is the quantity of heat energyEnergy is the ability to do work. Energy exists in several forms such as heat, kinetic or mechanical energy, light, potential energy, and electrical energy. Units of Energy: The SI unit... More that is required to change a substance from one state to another at constant temperature and pressure.
The unit of latent heat is JouleJoule is the SI (International System of Units) unit of energy and work. It is equal to the amount of work done when a force of 1 newton displaces a mass... More (J), and the symbol used for latent heat is Q.
Specific Latent Heat of a Substance:
Specific latent heat of a substance can also be defined as the quantity of heat energy that is required to change a unit mass (1 kg) of the substance from one state to another without a change in temperature (constant temperature).
There are different types of specific latent heat, this includes specific latent heat of fusion, and specific latent heat of vaporization which we will be discussing. There is also specific latent heat of sublimation.
What is the difference between Latent heat and Specific heat?
Ans: Latent heat is the quantity of heat transfer during the change in state, whereas Specific heat is the quantity of heat required to change the temperature of unit mass by one degree.
Specific Latent Heat of Fusion:
You are viewing an excerpt of this Topic. Subscribe Now to get Full Access to ALL this Subject's Topics and Quizzes for this Term!
Click on the button "Subscribe Now" below for Full Access!
Subscribe Now
Note: If you have Already Subscribed and you are seeing this message, it means you are logged out. Please Log In using the Login Button Below to Carry on Studying!



Responses