Calcium is the fifth most abundant element in the earth’s crust. However, it is too reactive to occur freely in nature. The most common compound of calcium is calcium trioxocarbonate (IV), CaCO3.

It occurs as calcite, chalk, limestone, and marble. It also found as double trioxocarbonate (IV) in gypsum (CaSO4⋅ 2H2O), CaCO3.MgCO3 is found in oyster shells and coral, and in many other compounds.
Extraction:
Calcium is extracted by electrolysis of fused calcium chloride, CaCl2, a by-product of the Solvay process. During the extraction process, some calcium fluoride is added to lower the melting pointThe temperature at which a solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid. The melting point is usually defined as the point... More to about 650oC from 850oC.
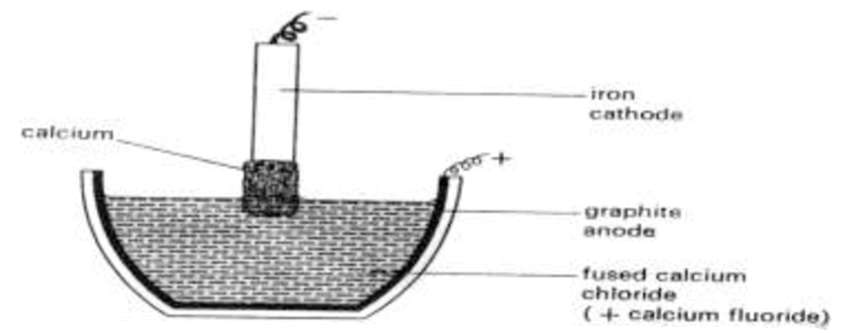
The mixture is then placed in a crucible which is lined on the inside with graphite. The graphite serves as the anodeAn anode is an electrode of a polarized electrical device through which conventional current enters the device. It is the positive part of electrolytes where oxidation takes place. More of the cell. The cathodeA cathode is the electrode from which a conventional current leaves the electrolyte. It is the negative part of the cell where reduction takes place. More is an iron rod with touches the surface of the electrolyteAn electrolyte is a substance that dissociates in water into charged particles called ions. Positively charged ions are called cations. Negatively charged ions are called anions. Simply, an electrolyte is a... More.
As electrolysis continues, the cathode collects metallic calcium. The cathode is gradually raised so that an irregular stick of calcium is formed on it. At the anode, chlorine gas is liberated.
At the cathode: The calcium receives two electrons each to become reduced to the metal.
Ca2+ + 2e– → Ca (reduction)
At the anode: Two chloride ions give up an electron each to become atomic chlorine. The two atoms then combine to become liberated as a gaseous molecule.
Cl– → Cl– + e–
Cl + Cl → Cl2
Overall electrolytic reaction:
Ca2(l) + 2Cl–(l) → Ca(s) + Cl2(g)
Test for Calcium Ions:
Flame test – Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. Calcium ions give an orange-red color in a flame test.
Procedure:
- A few drops of concentrated hydrochloric acid should be added to the unknown compound to moisten it.
- Dip the tip of a clean platinum wire into the mixture and hold it in a non-luminous Bunsen flame.
- Th unknown ions probably contain calcium ions If a bright brick red flame is produced.
- To confirm the presence of calcium ions, view the brick-red flame through a blue glass, the appearance of a green flame confirms the presence of calcium ions.
With sodium hydroxide – Add a few drops of NaOH solution to an unknown salt. If a white precipitate, insoluble in excess sodium hydroxide, forms, it indicates the presence of calcium ions.
Uses of Calcium:
- Calcium is used as a deoxidant in steel castings and copper alloys.
- It is used in the manufacture of calcium fluoride and calcium hydride.
- It is also used in the extraction of uranium.
- The biological use of calcium is to provide strength and structure to the skeleton. It is vital for the maintenance of bones and teeth.
- Gypsum (calcium sulfate) is used by builders as a plaster and by nurses for setting bones, as ‘plaster of Paris’.



Responses