(i) Electroplating: this is the coating of metals or objects with a layer of another metal. This method is used to protect and prevent the metal from rusting and to give an attractive appearance.
There are also specific types of electroplating such as copper plating, silver plating, and chromium plating. Electroplating allows manufacturers to use inexpensive metals such as steel or zinc for the majority of the product and then apply different metals on the outside to account for appearance, protection, and other properties desired for the product. The surface can be a metal or even plastic.
The material to be electroplated is the cathodeA cathode is the electrode from which a conventional current leaves the electrolyte. It is the negative part of the cell where reduction takes place. More and the anodeAn anode is an electrode of a polarized electrical device through which conventional current enters the device. It is the positive part of electrolytes where oxidation takes place. More is electroplating materials and the electrolytes will be the solution of the salt of the metals to be deposited.
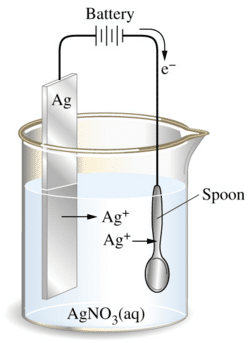
E.g. a silver spoon is electroplated by using a silver nitrate solution and pure silver as the anode. In the figure below, the Ag+ ions are being drawn to the surface of the spoon and it eventually becomes plated.
When current flows, Ag+, No3+, is produced, and Ag+ drifts to the cathode and is deposited on the spoon while the nitrate goes into solution.
(ii) Purification of metals
(iii) Extraction of metals
(iv) Production of gases
(v) Calibration of ammeter



Responses