A cell converts chemical energyEnergy is the ability to do work. Energy exists in several forms such as heat, kinetic or mechanical energy, light, potential energy, and electrical energy. Units of Energy: The SI unit... More into an electrical energy. There are two types of cells namely: primary cell and secondary cell
Primary Cell
A primary cell produces a current due to chemical components inside the cell. The chemicals are used up gradually as current is being produced and is non-reversible. Examples are Leclanche cell, Daniel cell.
Secondary Cells or Lead Accumulators
These are cells whose chemical reactions are reversible by passing current through them in opposite direction. They can be recharged after use. Examples are: lead-acid accumulators, alkaline battery. They have low resistance and can supply large current.
Simple Cell
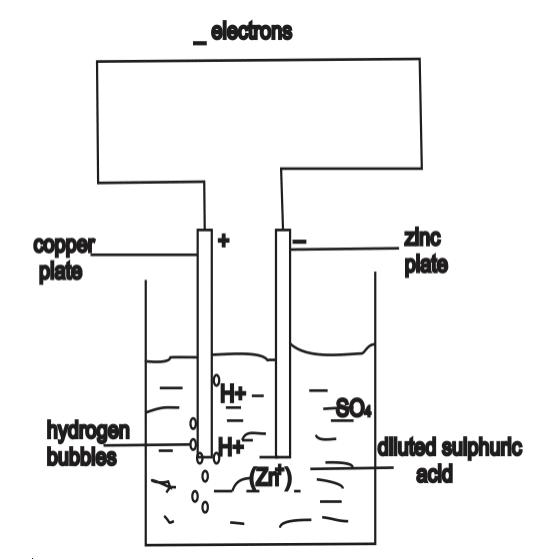
Simple cells consist of a copper plate and a zinc plate dipped in dilute sulphuric acid (tetraoxosulphate (VI) acid) contained in a vessel called Voltameter. When the pure zinc plate and the copper plate are joined together by a wire the zinc begins to dissolve bubbles of hydrogen are formed on the copper plate. A small electric bulb connected to the wire lights up showing that current is flowing in the wire. A voltmeter shows that copper plate is at higher potential than the zinc plate.
The copper plate is positive and the zinc plate is negative



Responses