Topic Content:
- Solids
- Liquids
- Gase
Solids:
The molecules in a solid are tightly packed together as a result of intermolecular forces of attraction that hold them together. A solid vibrates about its mean position and has definite shape and volume.
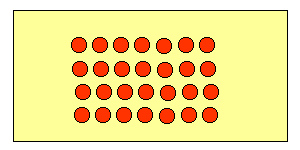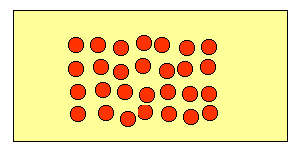
Liquids:
You are viewing an excerpt of this Topic. Subscribe Now to get Full Access to ALL this Subject's Topics and Quizzes for this Term!
Click on the button "Subscribe Now" below for Full Access!
Subscribe Now
Note: If you have Already Subscribed and you are seeing this message, it means you are logged out. Please Log In using the Login Button Below to Carry on Studying!



Responses