Topic Content:
- Definition of Nitrogen Cycle
- Ways by which Nitrogen is Added to the Soil in the Nitrogen Cycle
- Ways by which Soil can Gain Nitrogen
- Ways by which Soil Nitrogen is lost from the Soil
- Importance of Nitrogen Cycle
- Summary
A. What is the Nitrogen Cycle?
The nitrogen Cycle is a complex process through which nitrogen is converted into many forms, consecutively passing from the atmosphere, to the soil, to organisms and back into the atmosphere.
Nitrogen is naturally added and removed from the soil. It involves several processes such as nitrogen fixation, nitrification, denitrification, decay and putrefactionPutrefaction is the decay or rotting in the body or other organic matter by the action of microorganisms resulting in the production of a foul smell. It occurs between 10 to... More.
Ways by which Nitrogen is Added to the Soil in the Nitrogen Cycle:
- Direct fixation by lightening during rainfall (Electrical discharge)
- Nitrogen-fixing bacteria in the root nodules
 Root nodules are commonly found on the roots of leguminous plants e.g. peas, beans, soybean, alfalfa, clover, etc. They provide a home for symbiotic nitrogen-fixing bacteria, Rhizobium. Rhizobia is the general... More.
Root nodules are commonly found on the roots of leguminous plants e.g. peas, beans, soybean, alfalfa, clover, etc. They provide a home for symbiotic nitrogen-fixing bacteria, Rhizobium. Rhizobia is the general... More. - Ammonification.
- Nitrification.
- Decomposition of organic matterOrganic matter is anything that contains carbon compounds that were formed by living organisms. It covers a wide range of things like lawn clippings, leaves, stems, branches, moss, algae, lichens any... More.
- Application of nitrogenous fertilizers.
- Incorporation into the soil by free-living bacteria or non-symbiotic bacteria.
1. Electrical Discharge:
Nitrogen can be fixed into the soil during lightening. A lightning bolt, because of its high temperature, can break the bonds of atmospheric nitrogen molecules.
Free nitrogen atoms in the air combine with oxygen in the air to create nitrogen oxides, which dissolve in rainwater to form mild acids (nitrous and nitric acids), that are carried to Earth’s surface by precipitationPrecipitation is any liquid or frozen water that forms in the atmosphere and falls back to the Earth. It comes in many forms, like rain, sleet, and snow. More. These acids react with other compounds in the soil to form nitrates, very little nitrogen is fixed this way.
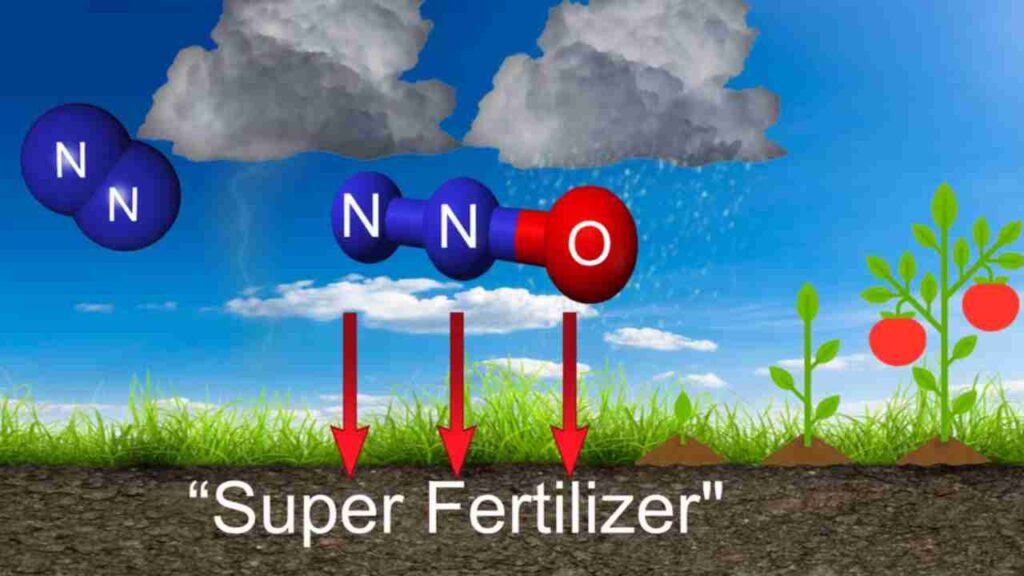
2. Ammonification and Nitrification:
You are viewing an excerpt of this Topic. Subscribe Now to get Full Access to ALL this Subject's Topics and Quizzes for this Term!
Click on the button "Subscribe Now" below for Full Access!
Subscribe Now
Note: If you have Already Subscribed and you are seeing this message, it means you are logged out. Please Log In using the Login Button Below to Carry on Studying!



Responses