
Sodium: Occurrence:
Sodium is the eighth most abundant element by weight in the earth’s crust. NaCl occurs in large amount in oceans, salt lakes and seas. Rock salt (NaCl) is the major source of sodium. It also occurs as soda feldspar (NaAlSi3O8), borax (Na2[B4O5(OH)4]·8H2O), chile saltpeter (NaNO3) and Glauber’s salt Na2SO4.10H2O.
Extraction of Sodium (Downs Process):
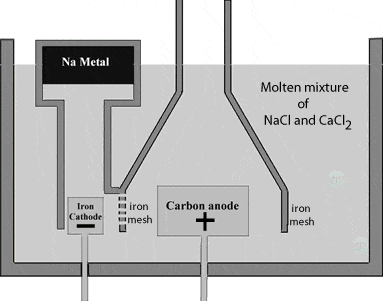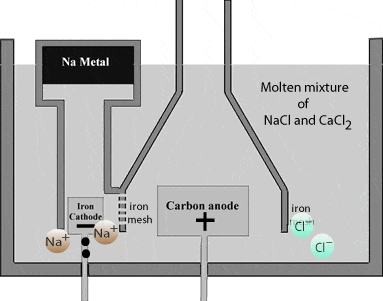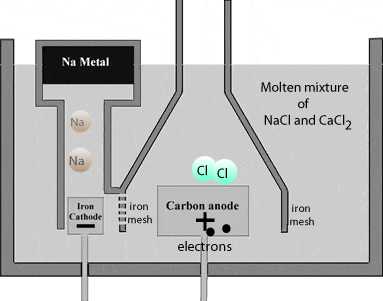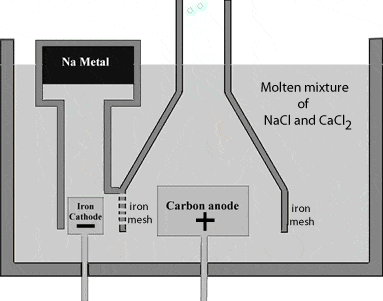
Sodium metal is extracted by Down’s process. This involves the electrolysis of fused sodium chloride. The anodeAn anode is an electrode of a polarized electrical device through which conventional current enters the device. It is the positive part of electrolytes where oxidation takes place. More is graphite and the cathodeA cathode is the electrode from which a conventional current leaves the electrolyte. It is the negative part of the cell where reduction takes place. More is a ring shaped iron. The two electrodes are separated by a wire gauge partition to avoid the mixing of sodium and chlorine formed.
Due to high melting pointThe temperature at which a solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid. The melting point is usually defined as the point... More of sodium chloride (about 800oC), a small amount of calcium chloride is added to the sodium chloride to lower its melting point to about 600oC.
Chlorine is liberated at the anode and escapes through the dome shaped steel hood outlet.
\( \scriptsize 2Cl^-_{(aq)} \: \rightarrow \: Cl_{2(g)} ^+ \: + \: 2e^-_{(aq)} ….(reduction)\)
At the cathode, sodium ion is attracted to the electrodeElectrodes are conductors, in the form of wires, rods or plates, through which current enters and leaves the electrolyte. When the current leaves the electrodes it is known as the cathode... More where they accept electrons and form sodium metal.
\( \scriptsize 2Na^+_{(aq)} \: +\: 2e^-_{(aq)} \: \rightarrow \: 2Na_{(s)}….(oxidation) \)
Overall reaction
\( \scriptsize 2Na^+ \: + \: 2Cl^-\: \: \rightarrow \: 2Na^+_{(s)} \: + \: Cl_{2(g)} \)
Sodium metal being lighter than the electrolyteAn electrolyte is a substance that dissociates in water into charged particles called ions. Positively charged ions are called cations. Negatively charged ions are called anions. Simply, an electrolyte is a... More, floats on the surface of the molten NaCl and flows into a separate container.
Advantages of Down’s Process:
1. Sodium metal obtained has high degree of purity (99.5%).
2. The raw material, sodium chloride is very cheap.
Physical Properties of Sodium:
i. Sodium is a silvery-white metal.
ii. It is quite malleable and soft.
iii. It is a good conductor of heat and electricity.
iv. Its melting point is 97°C.
v. It has the densityDensity is the measurement of how tightly a material is packed together i.e. how closely the particles are packed in the material. The tighter the material is packed the more its... More of 0.97 g/cm3
Chemical Properties of Sodium:
i. In air, sodium is swiftly oxidized to sodium oxide.
4Na + O2 → 2Na2O
ii. Reaction of sodium with dilute acids
2Na + 2HCl → 2NaCl + H2(g)
iii. Reaction with water: Na reacts vigorously with water to liberate hydrogen gas
2Na(s) + H2O → 2NaOH(aq) + H2(g)
iv. Reaction with ammonium gas: Sodium reacts with ammonia to form sodamide and hydrogen gas.
2Na + 2NH3 → 2NaNH2 + H2
v. Reaction with halogens: Sodium reacts with halogens to form salts.
2Na + Cl2 → 2NaCl
2Na + Br2 → 2NaBr
vi. Sodium reacts poorly with nitrogen to form sodium nitride.
6Na + N2 → 2Na3N
Uses of Sodium:

1. Sodium vapour lamps are used for lightening highways and airports.
2. Liquid sodium is used in nuclear reactors as a coolant.
3. Sodium is used in the manufacture of many compounds such as sodium peroxide, soda-lime, and sodium cyanide.

4. It is a good reducing agent for some chemical processes, like the extraction of titanium.



Responses