An electrolyteAn electrolyte is a substance that dissociates in water into charged particles called ions. Positively charged ions are called cations. Negatively charged ions are called anions. Simply, an electrolyte is a... More contains positive and negative ions. The molecules that constitute the ions will split in solution to the ions through electrolytic dissociation, irrespective whether an electric field is applied.
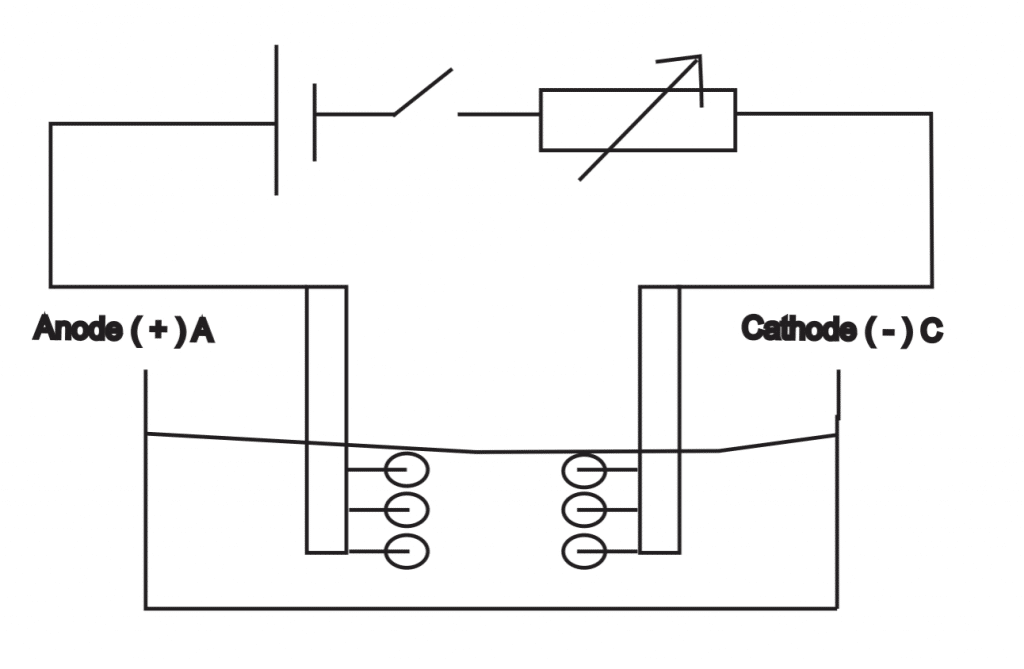
When the electrodes of a voltameter are connected to a battery, a Potential difference is set up, such that the positive ions move towards the cathodeA cathode is the electrode from which a conventional current leaves the electrolyte. It is the negative part of the cell where reduction takes place. More and negative ions drift towards the anodeAn anode is an electrode of a polarized electrical device through which conventional current enters the device. It is the positive part of electrolytes where oxidation takes place. More.
The directional movement of ions is the electric current flowing through the electrolyte. The electrolytic solution can conduct electricity due to the fact that electrolytes in the solution can conduct dissociates into ions



Responses