Topic Content:
- Definition of Charles Law
- Graphical Representation of Charles Law
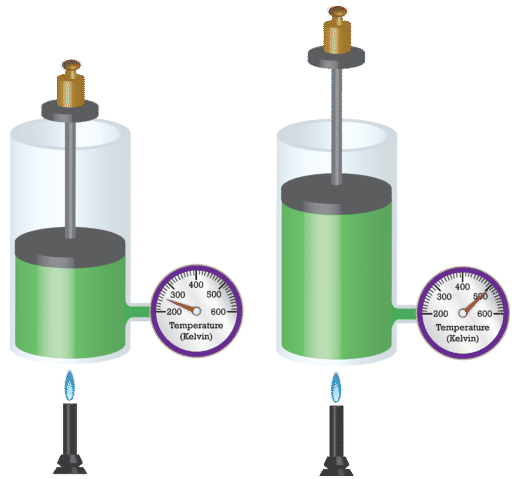
- Pictorial Representation of Charles Law
- How Kinetic Theory Explains Charles Law
- Calculations Based on Charles Law
A French chemist, Jacques Charles (1746-1823) found the relationship between the volume and temperature under constant pressure.
Charles law states that the volume of a given mass of gas is directly proportional to its absolute temperature or temperature in Kelvin, provided pressure remains constant.
It means that the volume of the gas increases as temperature increases and also decreases as temperature decreases i.e. (vice versa).
Mathematically,
You are viewing an excerpt of this Topic. Subscribe Now to get Full Access to ALL this Subject's Topics and Quizzes for this Term!
Click on the button "Subscribe Now" below for Full Access!
Subscribe Now
Note: If you have Already Subscribed and you are seeing this message, it means you are logged out. Please Log In using the Login Button Below to Carry on Studying!



Responses