Topic Content:
- Faraday’s Second Law of Electrolysis
- Verification of Faraday’s Second Law of Electrolysis
Faraday’s second law of electrolysis states that when the same quantity of electricity is passed through different electrolytes, the relative numbers of moles of element discharged are inversely proportional to the charges on the ion of the element respectively.
Whenever 1 mole of electrons is involved in an electrolytic cell, the total quantity of electricity involved is 96,500 Coulombs. This quantity is known as one Faraday and is denoted by F.
(i) 1 faraday of electricity is needed to discharge 1 mole of monovalent element during electrolysis:
Ag+(aq) + e– → Ag(s)
Na+(aq) + e– → Na(s) (1 mole of atom)
(ii) 2 faradays of electricity are needed to discharge 1 mole of divalent elementAn atom, with a valency of two, which thus can form two covalent bonds is called divalent. For example, oxygen (O), magnesium (Mg), etc. have a valency of two. More during electrolysis:
Cu2+(aq)+ 2e– → Cu(s)
Zn2+(aq) + 2e– → Zn(s) (2 moles of atoms)
(iii) 3 faradays of electricity are needed to discharge 1 mole of trivalent elementAn atom, ion, or element with a valence of three, is called trivalent. Examples of trivalent atoms are boron, aluminium, gallium, indium, etc. More during electrolysis:
Fe3+(aq) + 3e– → Fe
Al3+(aq) + 3e– → Al (3 moles of atoms)
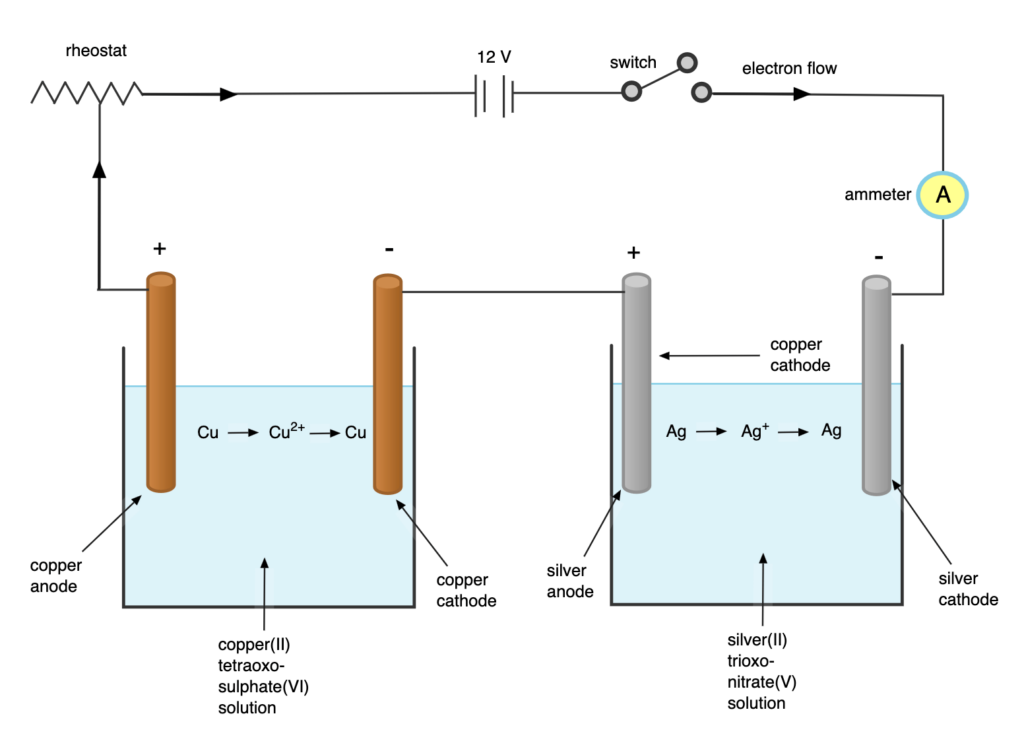
Verification of Faraday’s Second Law of Electrolysis:
Procedure: to verify Faraday’s second law of electrolysis, two electrolytic cells should be set up as shown in the image above:
You are viewing an excerpt of this Topic. Subscribe Now to get Full Access to ALL this Subject's Topics and Quizzes for this Term!
Click on the button "Subscribe Now" below for Full Access!
Subscribe Now
Note: If you have Already Subscribed and you are seeing this message, it means you are logged out. Please Log In using the Login Button Below to Carry on Studying!



Responses