In the previous topic, we discussed that electrons occupy energy levels and that the energy levels have sublevels. We also learned that sublevels have orbitals.
| n | Sublevels inside |
| 1 | s |
| 2 | s, p |
| 3 | s, p, d |
| 4 | s, p, d, f |
| 5 | s, p, d, f |
| Sublevel | Number of orbitals |
| s | 1 |
| s, p | 3 |
| s, p, d | 5 |
| s, p, d, f | 7 |
The only addition, compared to our table in the previous topic is a 5th energy level which contains s, p,d, and f sublevels. We can keep adding energy levels (6, 7, 8 ……with s, p, d, f sublevels) but we only need these 5 to determine the arrangement of electrons in each sublevel.
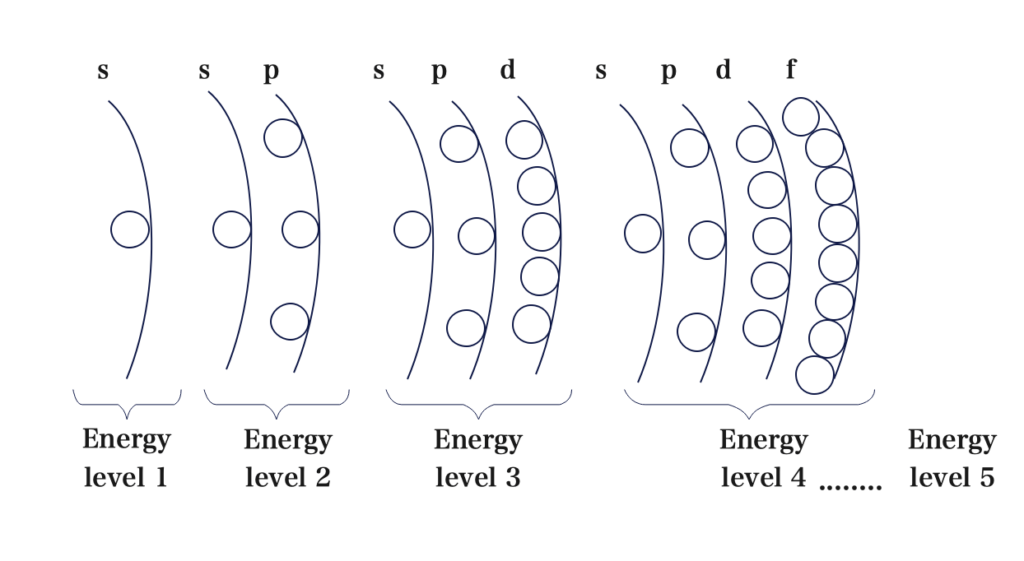
Electrons fill the orbitals according to a pattern rules or principles which we will discuss in this topic. Before we discuss the rules let’s first draw the following diagram.

The diagram above is just the energy levels and sublevels that are in each energy level.
Next what we have to do is draw arrows through 1s, and then 2s, then 2p and 3s then follow the pattern till we end with an arrow through 3d, 4p and 5s.
You are viewing an excerpt of this Topic. Subscribe Now to get Full Access to ALL this Subject's Topics and Quizzes for this Term!
Click on the button "Subscribe Now" below for Full Access!
Subscribe Now
Note: If you have Already Subscribed and you are seeing this message, it means you are logged out. Please Log In using the Login Button Below to Carry on Studying!



Responses